Titration again, woo hoo! Except this time, instead of using sodium hydroxide the compound we used to titrate was bright purple!! The compound was potassium permanganate, or KMnO4. And as with most times, I got the iron sample, thirteen. :)
First titration, fairly good color here!
Above is the entire redox equation for the lab.
Second Titration, even better color! You want the lightest permanent color change when titrating.
Titration involved swirling, and swirling, and swirling and swirling...
Phosphoric acid! We only needed 3 mL for each titration.
Look how super purple that stuff is, I love the oil slick appearance on the bottom of the open jar.
Above is just some random glassware that was in the disposal hood that I thought looked cool all together.
And there you have it! I hope you enjoyed your dose of science-ey goodness. :)



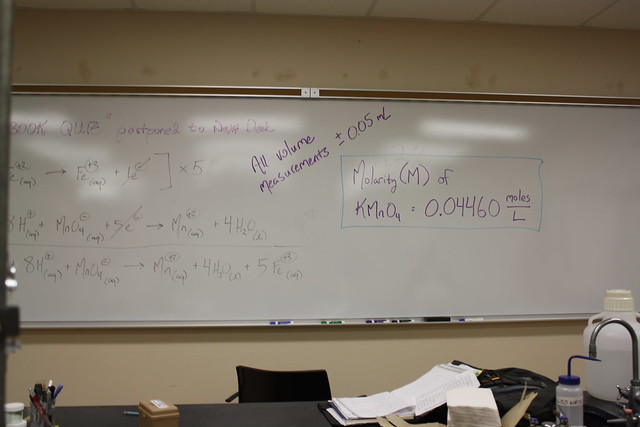
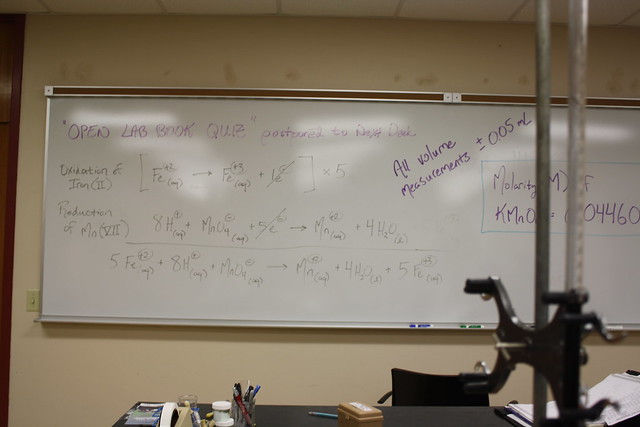










YAY, Science! I <3 it's face (and yours, duh!).
ReplyDeletewomen in stem
ReplyDelete