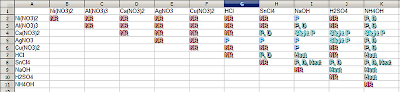
NR means No Reaction, P-Precipitate, 'P, D'- Precipitate then Dissolves, Slight P- Slight Precipitate
This was our first lab day, it took us a minute to compare each and every cation and anion to see what would happen in every reaction to identify if there would be precipitate (A solid substance formed between our two aqueous solutions), slight precipitate, or would form a precipitate and then dissolve as we added more of a solution with their ligands (something that forms a complex with the solution and basically prevents it from precipitating). We also knew that all of our bases and our acids would create heat, with no precipitate. Another identifier we were looking for was smoke, which is a reaction that occurs between HCl and NH4OH.
This was my groups set of chemicals. We had HCl-hydrochloric acid, H2SO4-Sulfuric Acid, SnCl4-Tin Chloride which was in a HCL solution, NaOh-Sodium Hydroxide, Ni(NO3)2-Nickel Nitrate, Al(NO3)3 Aluminum Nitrate, AgNO3- Silver Nitrate, Ca(NO3)2-Calcium Nitrate, Cu(NO3)2- Copper Nitrate , and NH4OH-Ammonium Hydroxide.
This was the other set, I have no idea what they are. :)
This was our first test. We had to determine the solutions with at least five steps. This was my thought process, but we discussed everything as a group. First, I wrote down what each of the three solutions that we knew were by direct observation. Then I wrote down what each of the solutions reacted with. The nickel nitrate and the copper nitrate only reacted with sodium hydroxide and ammonium hydroxide. We decided to test with copper nitrate. Since we also already knew the identity of ammonium hydroxide and nickel nitrate, we only needed to test the other 7 solutions. We added each unknown solution into it's own test tube, then added a little copper nitrate to each and looked for a precipitate. What was interesting is that the precipitate took all the blue out of the solution! Really cool looking. Here's my really bad paint example.
Above is supposed to be adding copper nitrate, which is blue, to 7 clear solutions. All of the samples except sodium hydroxide would just turn a diluted blue. Sodium hydroxide however would form a blue precipitate, taking all of the color out of the solution. Next we wanted to find out which were the acids. We also had the tin chloride which was in a hydrochloric solution, but we also knew that would form a slight precipitate. Hydrosulfuric acid and hydrochloric acid would just create heat with sodium hydroxide. We were also able to identify silver in this test because it creates a brown precipitate.Then to identify what which of the two solutions, that created heat when mixed, were which, we mixed them both with ammonium hydroxide. As I mentioned before, one of our identifiers was that hydrochloric acid and ammonium hydroxide would create smoke. The last two solutions we needed to identify would be aluminum nitrate and calcium nitrate, and to decide which was which, we added more sodium hydroxide to each of the unknown test tubes. Both form a precipitate with sodium hydroxide, but when enough is added to aluminum nitrate, then the precipitate disappears, where as in calcium nitrate the precipitate will be murky, but will still persist.
So there you have it! The super boring post of how to identify ten liquids by their reactions and by direct observation. :)


No comments:
Post a Comment